
Comparative analyses were performed to explore lineage-specific changes in gene expression, subpopulation composition, and intercellular communication in HCM cardiac tissues. We also conducted spatial transcriptomic assays on cardiac tissue sections from HCM patients. In this study, we performed snRNA-seq of the cardiac tissues from HCM patients and healthy donors. Integrated analysis of snRNA-seq and spatial transcriptomic data would profoundly improve our knowledge regarding the cellular and molecular changes of HCM. The recent advent of spatially resolved transcriptomics has greatly expanded our scope and power to study the pathogenesis mechanism of diseases by providing spatial information of gene expression that is lost in single-cell/nucleus data 10. However, there is still a lack of research to explore the transcriptomic changes in cardiac interventricular septum (IVS) under the diseased condition of HCM at single-nucleus resolution. snRNA-seq has been successfully applied to dissect the heterogeneity of the adult human heart under healthy conditions 9. Single-cell or single-nucleus RNA-seq (snRNA-seq) can overcome this limitation and allows for unbiased dissection of the cellular changes at unprecedented resolution. However, cell-type-specific changes could not be detected from bulk data. Previous studies have employed bulk RNA-seq to explore the transcriptomic alterations in the cardiac tissue of HCM at the tissue level 7, 8. An in-depth elucidation of the cellular and molecular changes in pathological cardiac remodeling of HCM is pivotal for developing medical therapies to successfully prevent or mitigate the HCM progression.
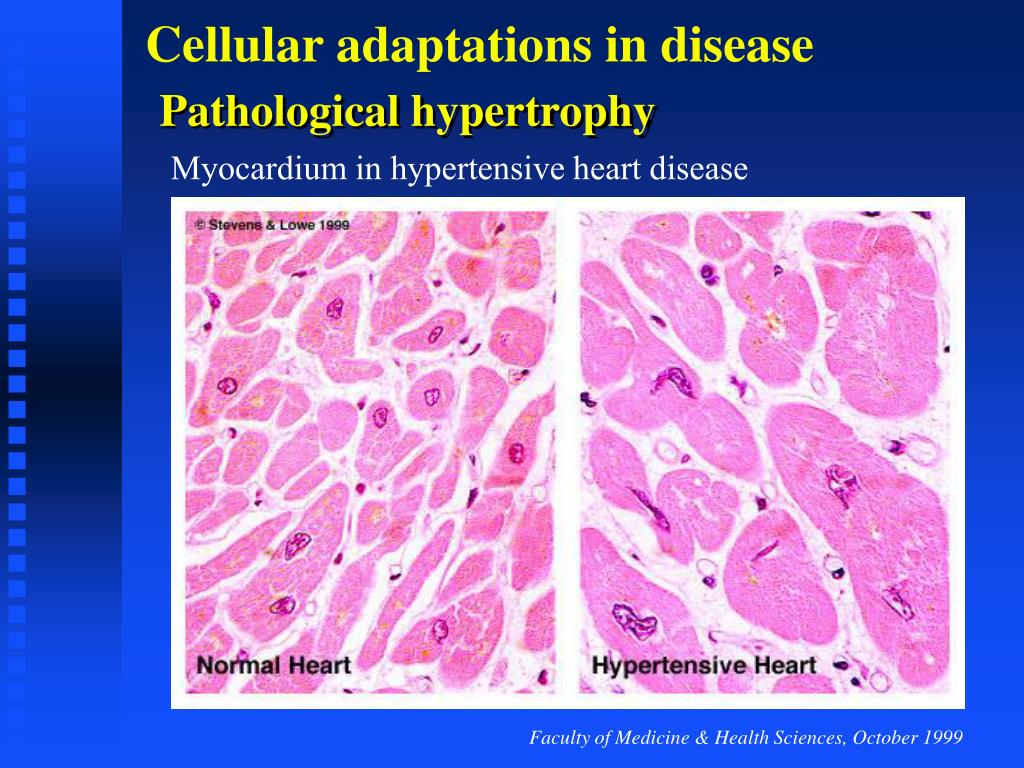
However, effective targeted drugs for HCM are still very limited. MYK-461, for instance, inhibits cardiac myosin ATPase 6. In recent years, significant efforts have been made to develop therapeutic agents for HCM. If left untreated, pathological cardiac remodeling may lead to adverse events, including heart failure, arrhythmias, and death. Pathological cardiac remodeling occurs in the myocardium of HCM patients 5, manifesting as cardiomyocyte dysfunction, escalated fibroblast activation (fibrosis), chronic inflammation, and cell death. Cardiomyocyte hypertrophy and disarray, and cardiac fibrosis are the key histopathological hallmarks of HCM 4. HCM is characterized by an increased left ventricular wall thickness in the absence of an associated cardiac or systemic disease 3. HCM is the leading cause of sudden cardiac deaths (SCDs) in young people, accounting for 36% of SCDs in young athletes 2. Hypertrophic cardiomyopathy (HCM) is the most common cardiac genetic disorder with an estimated prevalence of 1 in 200 1. Our study provided a comprehensive analysis of the lineage-specific regulatory changes in HCM, which laid the foundation for targeted drug development in HCM. Moreover, we showed experimental evidence that in vitro knockdown of AEBP1 could promote the activation of human cardiac fibroblasts, and overexpression of AEBP1 could attenuate the TGFβ-induced activation. Using the spatial transcriptomic data, spatial activity patterns of the candidate genes, pathways, and subpopulations were confirmed on patient tissue sections. Transcriptomic dynamics underlying cardiac fibroblast activation were also uncovered, and potential key genes involved in cardiac fibrosis were obtained such as AEBP1, RUNX1, MEOX1, LEF1, and NRXN3. According to the results of pseudotime ordering, differential expression analysis, and differential regulatory network analysis, potential key genes during the transition towards a failing state of cardiomyocytes such as FGF12, I元1RA, and CREB5 were identified. Lineage-specific changes in gene expression, subpopulation composition, and intercellular communication in HCM were discovered through comparative analyses. Unbiased clustering of 55,122 nuclei from HCM and healthy conditions revealed 9 cell lineages and 28 clusters. Here, we performed single-nucleus RNA-seq of the cardiac tissues from HCM patients or healthy donors and conducted spatial transcriptomic assays on tissue sections from patients. An in-depth elucidation of the lineage-specific changes in pathological cardiac remodeling of HCM is pivotal for the development of therapies to mitigate the progression. Pathological cardiac remodeling in the myocardium of HCM patients may progress to heart failure. Hypertrophic cardiomyopathy (HCM) is the most common cardiac genetic disorder characterized by cardiomyocyte hypertrophy and cardiac fibrosis.


 0 kommentar(er)
0 kommentar(er)
